Abstract
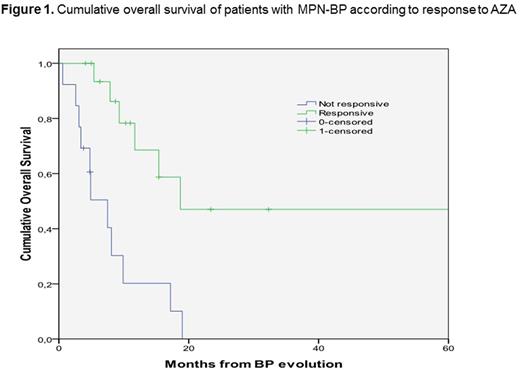
Treatment options for patients in blastic phase following evolution of a myeloproliferative neoplasm (MPN-BP) are limited and the overall prognosis is very poor with a survival likelihood of only a few months. There are some reports on a possible role of azacytidine (AZA), but the results are conflicting and no prognostic factors are at present available. In the present study, we focused on the identification of prognostic factors for response and overall survival (OS) in a cohort of 32 patients - M/F 21/11, median age at evolution 70.4 years, interquartile range (IQR) 64.2-77.1 - with MPN-BP treated with AZA and collected in the retrospective database of the Latial cooperative study group. The primary MPN diagnosis was essential thrombocythemia in 10 cases (31.3%), policythemia vera in 8 (25%), primary myelofibrosis in 10 (31.3%) and myelodysplastic syndrome (MDS)/MPN in 4 (12.4%). The JAK-2 V617F mutation was present in 20/29 patients tested (68.9%). All but 2 patients had received previous chemotherapy during the chronic phase. Median time from diagnosis to BP evolution was 61.4 months (IQR 25.5-168.1). All patients were treated with AZA at the standard dose of 75 mg/m2: 29 patients received a 7-day schedule and 3 a 5-day schedule every 28 days. Two patients are too early for response evaluation. Among the remaining 30 patients, 2 died early (6.6%) after AZA initiation due to pulmonary fungal infection and respiratory failure, respectively, 11 patients (36.7%) had a disease progression (DP) or stable disease (SD), 5 patients (16.7%) had a hematological improvement (HI), 4 patient (13.3%) achieved a partial remission (PR) and 8 patients (26.7%) a complete remission (CR): overall, 17/30 patients (56.7%) responded to treatment (HI + PR + CR) with AZA. The median overall survival (OS) from BP evolution of the whole cohort was 11.7 months (95% CI 3.5-19.8). There was no difference in the median OS among patients with HI, PR or CR (p >0.908): thus, we considered together these latter three groups for the analysis of prognostic factors as "responsive patients", with a median OS of 18.7 months (95% CI 0-65.9) compared to 7.5 months (95% CI 3.6-11.3) in non-responding patients (p=0.001) (Fig. 1). The following features were analyzed as prognostic factors for response achievement and OS: gender, age at evolution, peripheral blood values at evolution, type of previous MPN, JAK-2V617F positivity. Only female sex was significant for both achievement of response (0.009) and OS duration (p=0.001). In conclusion, AZA is useful for the treatment of MPN-BP, with an overall response rate of 56.7% (17/30 patients) and an advantage in OS for responsive patients. In our cohort, only gender showed a prognostic impact, but a more refined prognostic stratification needs to be performed on a larger number of patients.
Breccia: Bristol Myers Squibb: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Novartis: Consultancy. Foà: Roche, Janssen, Gilead, Amgen, Celgene, BMS, Sandoz, Novartis, AbbVie: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.